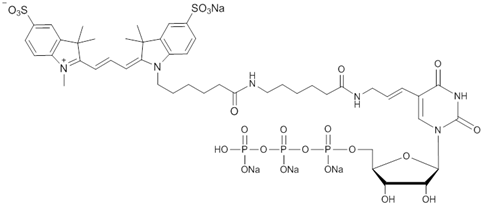
Cy3-X-UTP (cat. no. C420) is supplied as a 1 mM solution in TE buffer.
Cy3-X-UTP can be enzymatically incorporated into RNA with T7, T3 and SP6 RNA polymerases, as well as other RNA modifying enzymes. The X linker between Cy3 and UTP can improve the incorporation efficiency.
|
Specifications:
Excitation/Emission (nm): 553/565 nm
|
|
Applications
Indirect non-radioactive enzymatic labeling of RNA during in vitro transcription.
| Protocol (PDF): | C420 |
| MSDS (PDF): | MSDS-C420 |
Reference:
High-density polymerase-mediated incorporation of fluorochrome-labeled nucleotides.
Ramanathan A, Pape L, Schwartz DC
Anal Biochem (2005) 337:1-11
Sensitive fluorescence in situ hybridization signal detection in maize using directly labeled probes produced by high concentration DNA polymerase nick translation.
Kato A, Albert PS, Vega JM, Birchler JA
Biotech Histochem (2006) 81:71-78
Visualizing chromatin and chromosomes in living cells.
Zink D, Sadoni N, Stelzer E
Methods (2003) 29:42-50
Frequently Asked Questions (FAQs)
I'm getting high background after labeling with fluorescent nucleotides. What do you recommend I do?
You can try to purify the fluorescent labeled probe with an appropriate spin column-based method to remove unincorporated fluorescent nucleotides. Ethanol precipitation may not efficiently remove the unincorporated fluorescent nucleotides, so a spin column will need to be used.
Can fluorescent nucleotides be used for labeling nucleic acids in live cells?
No, they are not cell permeant so they are only suitable for in vitro incorporation methods. The fluorescent dyes and phosphate groups are too highly charged to allow the nucleotides to penetrate the membrane of an intact cell. Nonfluorescent nucleosides without phosphates such as EdU, EU, or BrdU can be used for live cell nucleic acid incorporation studies.
How do I determine the incorporation efficiency of the fluorescent nucleotides after enzymatic incorporation?
The base-to-dye ratio is determined by measuring the absorbance of the nucleic acid at 260 nm and the absorbance of the dye at its absorbance maximum. Using the extinction coefficients for the appropriate dye and nucleic acid, you can then calculate the base-to-dye ratio for the labeled nucleic acid using the Beer-Lambert law.
Can PCR be used to incorporate fluorescent dUTPs?
With PCR, incorporation rates are very low.
What methods can I use to enzymatically incorporate fluorescent nucleotides?
Fluorescent dUTPs are most efficiently incorporated into DNA using nick translation, random primer labeling, end-labeling with terminal deoxynucleotidyl transferase (TdT), and reverse transcription. Fluorescent UTPs can be incorporated into RNA using in vitro transcription.
Where is the fluorescent dye attached on the fluorescent dUTP and UTP?
The fluorescent dUTP and UTP nucleotides are modified at the C-5 position of uridine via a unique allylamino linker.
Can dU-containing DNA produced by amplification with dUTP be cut by restriction enzymes?
You will need to confirm with the manufacturer of the restriction enzyme you are using to find out whether or not it can recognize dUTP-containing DNA.
The nucleic acid probe is not fluorescent after labeling with fluorescent nucleotides. What do you recommend I try?
- Check the base-to-dye ratio to determine the level of incorporation of the fluorescent nucleotides. Since fluorescent detection may be affected by underlabeling, overlabeling, instrument sensitivity, or other factors, the base-to-dye ratio is a better indicator of incorporation efficiency.
- Fluorescent nucleotides may not have been incorporated well in the enzymatic labeling reaction. Make sure that the enzymatic method used is compatible with the particular fluorescent nucleotide, since some methods may not be appropriate for all applications. You may also need to further optimize the enzymatic incorporation method, for example by optimizing enzyme concentration, incubation time, concentration, and ratio of labeled and unlabeled nucleotides. For PCR, a lower fidelity polymerase may give higher incorporation rates; however, incorporation rates will be generally low using PCR.
- Check the fluorescent filter used for detection to make sure it is compatible with the dye. You can also test a small drop of the undiluted fluorescent nucleotide in your filter to make sure you can image the dye alone before it is conjugated to the oligonucleotide.
Related Products
- 5-Aminoallyl-dUTP, 10 mM in TE buffer
- 5-Aminoallyl-UTP, 10 mM in TE buffer
- Biotin-11-dUTP, 1 mM in TE buffer
- Biotin-11-UTP, 1 mM in TE buffer
- 5-FAM-dUTP, 1 mM in TE buffer
- 5-FAM-X-dUTP, 1 mM in TE buffer
- 5-FAM-UTP, 1 mM in TE buffer
- 5-FAM-X-UTP, 1 mM in TE buffer
- Andy Fluor 488-X-dUTP, 1 mM in TE buffer
- Andy Fluor 488-X-UTP, 1 mM in TE buffer
- Andy Fluor 555-X-dUTP, 1 mM in TE buffer
- Andy Fluor 555-X-UTP, 1 mM in TE buffer
- Andy Fluor 568-X-dUTP, 1 mM in TE buffer
- Andy Fluor 568-X-UTP, 1 mM in TE buffer
- Andy Fluor 594-X-dUTP, 1 mM in TE buffer
- Andy Fluor 594-X-UTP, 1 mM in TE buffer
- Andy Fluor 647-X-dUTP, 1 mM in TE buffer
- Andy Fluor 647-X-UTP, 1 mM in TE buffer
- Cy3-X-dUTP, 1 mM in TE buffer
- Cy3-X-UTP, 1 mM in TE buffer
- Cy5-X-dUTP, 1 mM in TE buffer
- Cy5-X-UTP, 1 mM in TE buffer
